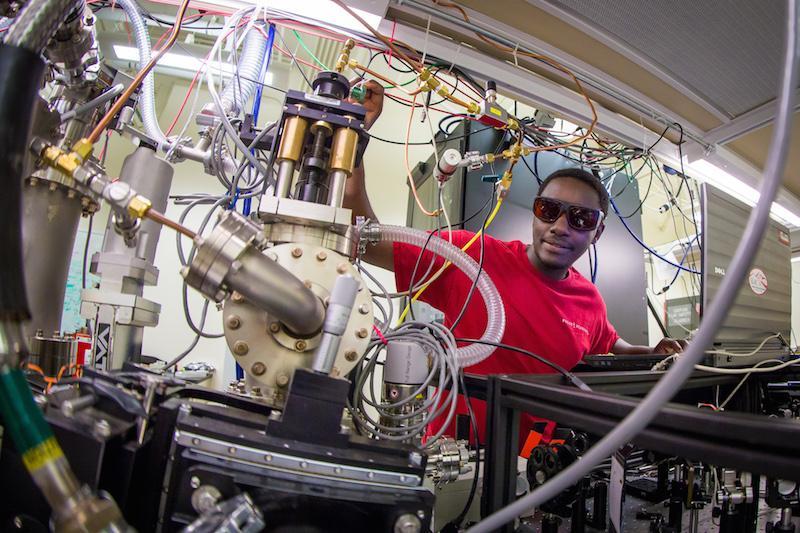
PULSE Institute

One of the research foci of the Chemical Science Division is the study of Ultrafast Chemical Science at the PULSE Institute, a research institute within the Science Directorate of SLAC as well as a Stanford Independent Laboratory. The motion of atoms inside molecules during a chemical transformation, or the atomic motion that accompanies a phase change from solid to liquid occurs very fast—on the order of one trillionth of a second, or a picosecond. The electrons that rearrange in bonds when light is absorbed or during a chemical transition can move more than one thousand times faster than that—on the order of a femtosecond or less. At SLAC, scientists are able to utilize the LCLS (Linac Coherent Light Source), a “camera” that employs an X-ray beam, to capture these motions and reactions with time resolutions in the femtosecond range.
Some of our major research interests include:
- Imaging Science
- Nanostructure determination
- Light Conversion Chemistry
- Photocatalysis in coordination complexes
- 2D systems and interfaces
- Fundamental Attoscale Science
While LCLS is a primary tool for our researchers, PULSE' mission is tied to LCLS research and it provides the local infrastructure for LCLS users and scientists. PULSE is also active in LCLS and SSRL (Stanford Synchrotron Radiation Lightsource) instrument commissioning, and develops tools and protocols for the two X-ray facilities.
In addition to scientific research, PULSE also provides education in Ultrafast X-ray Science through programs such as the annual Ultrafast X-ray summer school, which has introduced hundreds of students and postdocs to LCLS research.
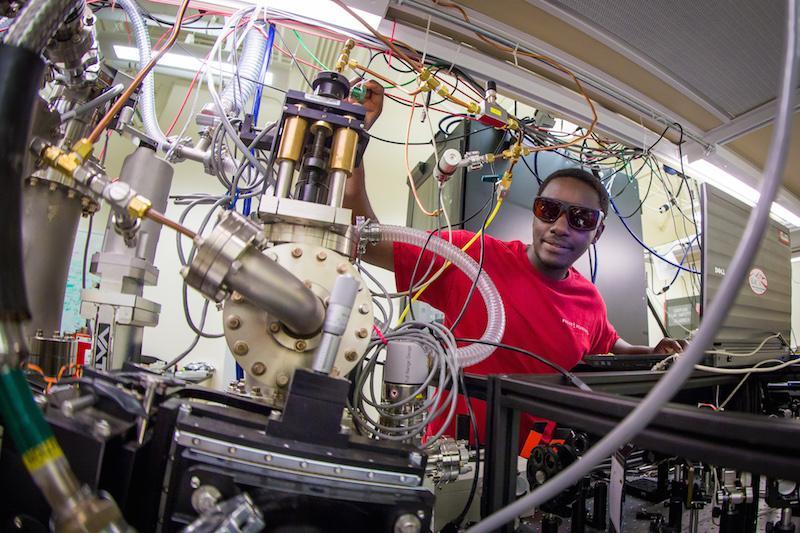
2016_0601_georges_ndabashimiye_photos_7923.jpg
For the experiment, Stanford graduate student Georges Ndabashimiye had to figure out how to freeze argon gas into a thin layer inside a small vacuum chamber chilled to 20 kelvins - close to absolute zero. (SLAC National Accelerator Laboratory)